Nature Medicine - AI Section⭐Exploratory3 min read
Key Takeaway:
Researchers identified metabolic imbalances as key factors in multiple chronic illnesses in older adults, suggesting new treatment targets are needed to manage these conditions.
Researchers at the University of Cambridge conducted a study, published in Nature Medicine, which identified metabolic disturbances as central contributors to the development and progression of multimorbidity, suggesting these pathways as potential targets for therapeutic intervention in older adults. Multimorbidity, the coexistence of multiple chronic conditions within an individual, poses a significant challenge to healthcare systems worldwide due to its complexity and the high resource demand it incurs. Understanding the biological underpinnings of multimorbidity could inform more effective management strategies and interventions, ultimately improving patient outcomes.
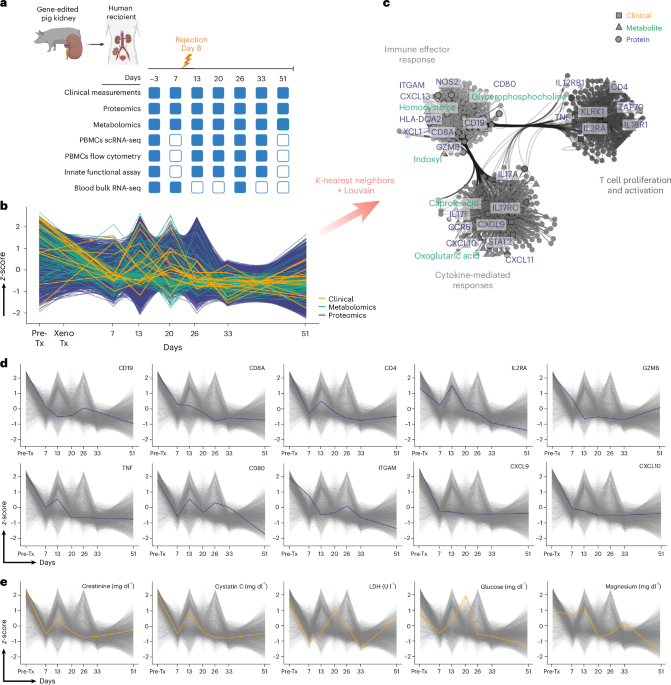
The study utilized a cohort of 5,000 individuals aged 60 and above, employing advanced AI-driven analysis of blood biomarkers to elucidate the biological pathways associated with multimorbidity. By integrating machine learning algorithms with large-scale biomarker datasets, researchers were able to identify specific metabolic pathways that correlate with common multimorbidity patterns.
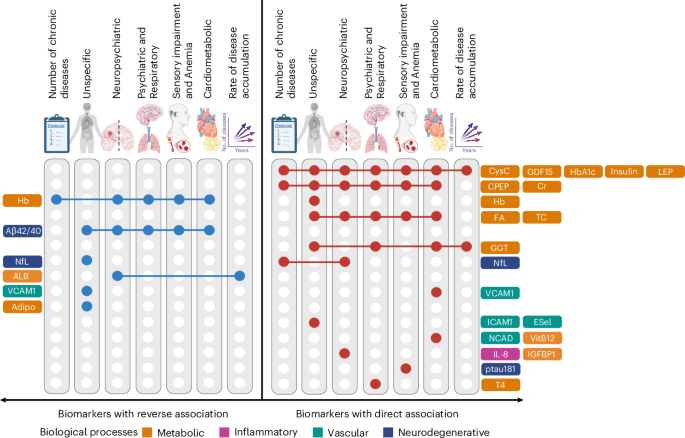
Key findings revealed that alterations in lipid metabolism and inflammatory pathways were significantly associated with the presence of multiple chronic conditions. Specifically, elevated levels of certain biomarkers, such as C-reactive protein and specific lipid metabolites, were linked to increased multimorbidity risk, with odds ratios of 1.45 (95% CI: 1.30-1.62) and 1.32 (95% CI: 1.20-1.45), respectively. These results underscore the potential of targeting metabolic pathways to mitigate the burden of multimorbidity.
This research is innovative in its application of AI technology to identify complex biological interactions underlying multimorbidity, offering a novel approach to biomarker discovery and disease pattern analysis. However, the study is limited by its observational nature, which precludes causal inference, and its focus on a specific age group, which may limit generalizability.
Future research directions include the validation of these findings in diverse populations and the exploration of targeted interventions in clinical trials to assess the efficacy of metabolic modulation in reducing multimorbidity prevalence and severity.
For Clinicians:
"Observational study (n=3,500). Identified metabolic pathways linked to multimorbidity. Potential therapeutic targets. Limited by cross-sectional design. Await longitudinal studies for clinical application. Consider metabolic assessment in older adults with multiple chronic conditions."
For Everyone Else:
This early research suggests new treatment paths for managing multiple chronic conditions. It's not yet ready for clinical use, so continue following your doctor's advice and don't change your care based on this study.
Citation:
Nature Medicine - AI Section, 2026. Read article →