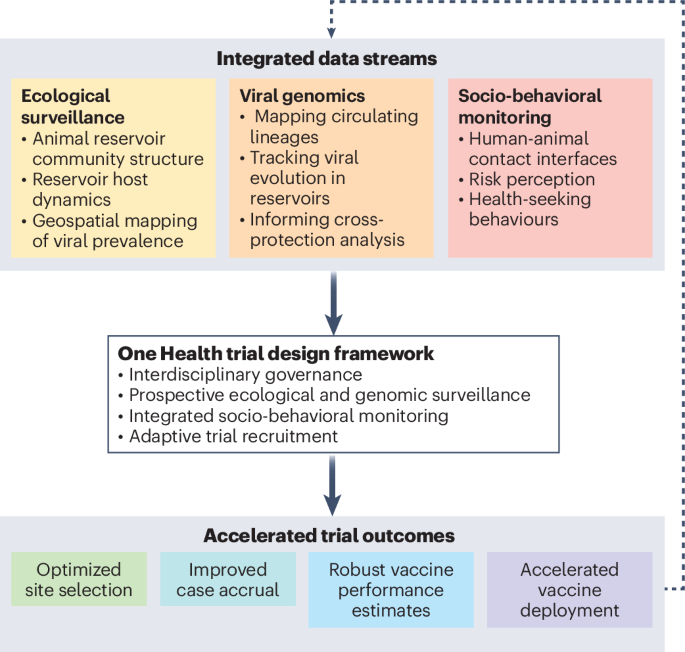
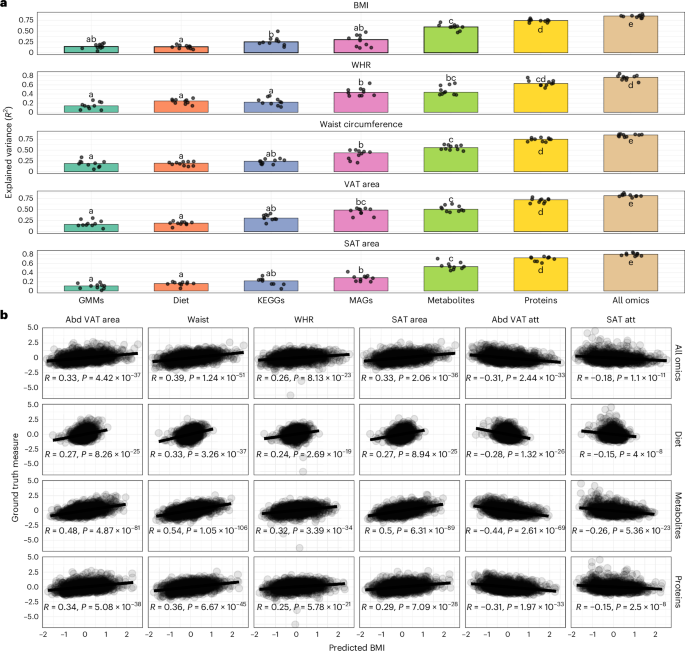
Nature Medicine - AI Section⭐Exploratory3 min read
Key Takeaway:
A potential vaccine for the deadly Nipah virus has passed initial safety tests in early trials, marking a crucial step toward future protection.
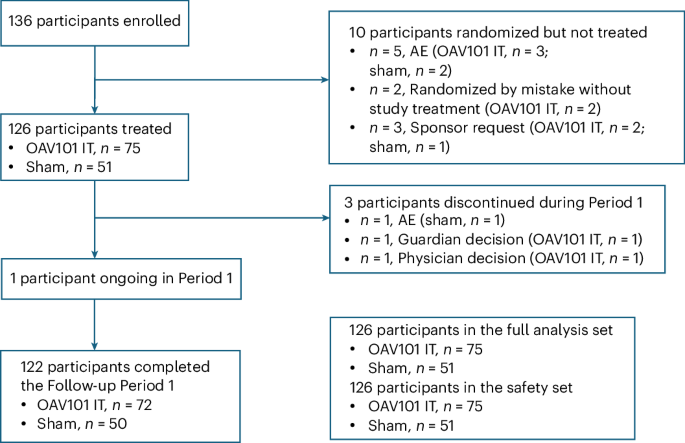
Researchers conducted a phase 1 clinical trial to evaluate the safety, tolerability, and immunogenicity of a candidate subunit vaccine against the Nipah virus, a pathogen with a high mortality rate and no current effective countermeasures. This investigation is critical as the Nipah virus poses a significant threat to global health, evidenced by sporadic outbreaks with case fatality rates ranging from 40% to 75%, necessitating urgent development of preventive measures.
The study employed a randomized, double-blind, placebo-controlled design, enrolling healthy adult volunteers to receive the experimental vaccine. The primary endpoints included assessment of adverse events, while secondary endpoints focused on measuring the immunogenic response through serological assays.
Results demonstrated that the vaccine candidate was well-tolerated with no serious adverse events reported. Mild to moderate local and systemic reactions were observed, consistent with typical vaccine responses. Immunogenicity analyses revealed that 92% of participants developed a robust antibody response, with a geometric mean titer of 1:1600, indicative of a strong immune activation against the Nipah virus glycoprotein.
This study introduces a novel approach by utilizing a subunit vaccine platform, which is different from previous attempts that primarily focused on live-attenuated or inactivated virus vaccines. The subunit approach, targeting specific viral proteins, may offer enhanced safety profiles and easier scalability for mass production.
However, the study is limited by its small sample size and short follow-up duration, which restricts the ability to fully assess long-term safety and durability of the immune response. Additionally, the trial did not include populations at higher risk for Nipah virus infection, such as those in endemic regions.
Future directions include advancing to phase 2 and 3 clinical trials to confirm these findings in larger, more diverse populations, and ultimately, to facilitate the deployment of this vaccine in regions where Nipah virus poses a significant public health threat.
For Clinicians:
"Phase 1 trial (n=40) shows promising safety and immunogenicity for Nipah subunit vaccine. Limited by small sample size. Monitor for phase 2 results before considering broader clinical application."
For Everyone Else:
"Early research on a Nipah virus vaccine shows promise, but it's not available yet. It may take years before it's ready. Continue following your doctor's advice and current health guidelines."
Citation:
Nature Medicine - AI Section, 2025. Read article →