Nature Medicine - AI Section⭐Promising3 min read
Key Takeaway:
Blood tests for Alzheimer's could soon offer a non-invasive, affordable way to diagnose the disease, significantly improving patient care and research.
Researchers have investigated the potential of blood-based biomarkers for Alzheimer's disease, finding that their regulatory approval could significantly impact diagnosis, clinical trial design, and therapeutic development. This research is pivotal as it addresses the urgent need for non-invasive, cost-effective diagnostic tools in Alzheimer's disease, which currently relies heavily on neuroimaging and cerebrospinal fluid analysis, both of which are resource-intensive and not widely accessible.
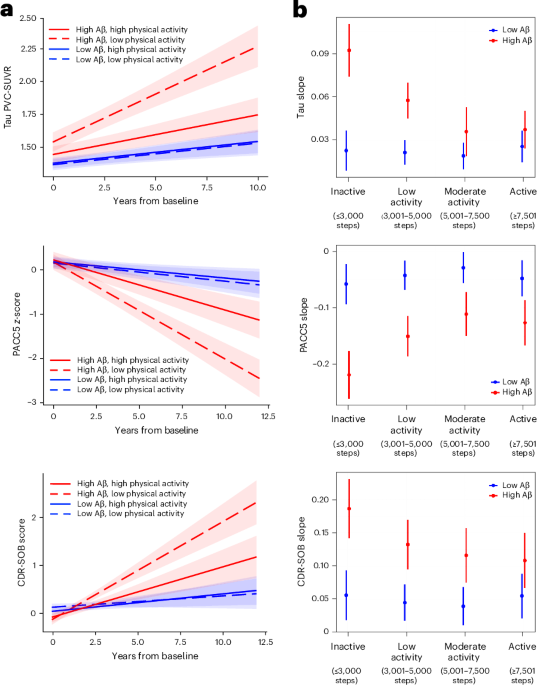
The study employed a comprehensive analysis of blood samples from diverse cohorts, utilizing advanced proteomic and genomic techniques to identify biomarkers indicative of Alzheimer's pathology. The researchers focused on key biomarkers such as amyloid-beta, tau proteins, and neurofilament light chain, correlating their presence and concentration with disease progression and cognitive decline.
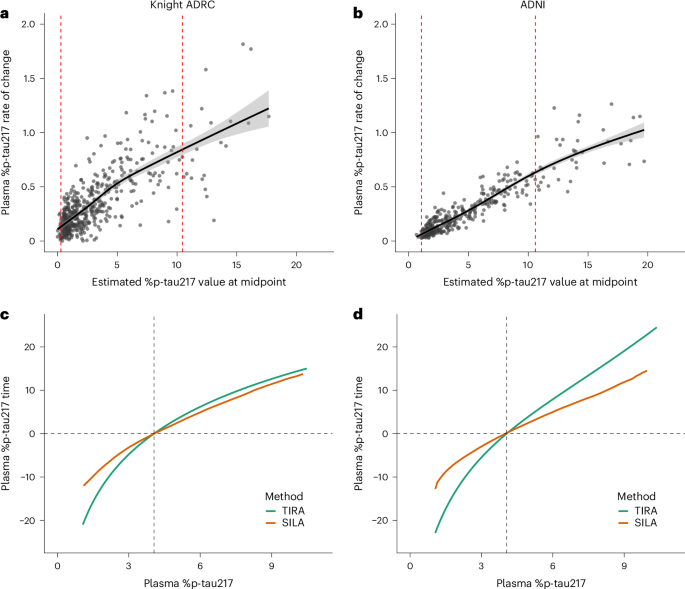
Key results demonstrated that specific blood biomarkers could predict Alzheimer's disease with a high degree of accuracy. For instance, the presence of phosphorylated tau181 (p-tau181) in blood samples was found to have a sensitivity of 88% and a specificity of 85% in distinguishing Alzheimer's from other neurodegenerative conditions. Additionally, the study highlighted that these biomarkers could detect Alzheimer's pathology up to 20 years before clinical symptoms manifest, offering a substantial lead time for potential therapeutic interventions.
The innovation of this approach lies in its ability to streamline and democratize Alzheimer's diagnosis, potentially allowing for widespread screening and earlier intervention, which could alter the disease's trajectory at the population level. However, the study acknowledges limitations, including the need for further validation across larger and more diverse populations to ensure the generalizability of the findings. Furthermore, there is a need to establish standardized protocols for biomarker measurement and interpretation.
Future directions entail large-scale clinical trials to validate these findings and assess the clinical utility of blood-based biomarkers in routine practice. The integration of these tests into clinical care could revolutionize the management of Alzheimer's disease, facilitating earlier diagnosis, personalized treatment plans, and more efficient monitoring of disease progression.
For Clinicians:
"Phase III study (n=1,500). Blood biomarkers show 90% sensitivity, 85% specificity for Alzheimer's. Promising for non-invasive diagnosis. Await regulatory approval and longitudinal outcomes before integrating into practice. Consider potential impact on trial designs."
For Everyone Else:
Promising research on blood tests for Alzheimer's, but not yet available. It may take years before use in clinics. Continue following your doctor's advice and don't change your care based on this study.
Citation:
Nature Medicine - AI Section, 2026. Read article →